Flat-Surface Grafting in Arabidopsis thaliana
Seung Y. Rhee* and Chris R. Somerville#
Biological Sciences Department
Stanford University
Stanford, CA 94305
Tel:(415)-325-1521 ext. 426
syrhee@Andrew.stanford.edu
#Carnegie Institution of Washington
Department of Plant Biology
290 Panama Street Stanford, CA 94305-1297
Abstract
We have developed an efficient method of grafting in Arabidopsis thaliana that uses flat-surface cutting and a capillary tube for support of the graft junction. Approximately 40% of grafts resulted in healthy plants that produced amounts of seed comparable to ungrafted plants.
Introduction
Grafting is joining parts of different plants in order to continue their growth as one plant. The plant that provides the root is called a rootstock and the added piece of another plant is called a scion (Janick, 1979). Grafting has been used since 1000 B.C. to modify the properties of horticultural species (Hartmann and Kester, 1975). In addition, grafting has been used to study various aspects of plant biology including plant pathology (Bertaccini and Bellardi, 1992), hormone action (Proebsting et al., 1992), mineral nutrition (Jayawickrama et al., 1992), apical dominance (Mapelli and Kinet, 1992), nodulation (Pedalino et al., 1992), flowering (Zeevaart, 1958), dwarfing (White et al., 1992) and characterization of mutants (Tsukaya et al., 1993). In the present paper, we describe a new method of grafting in Arabidopsis thaliana, which is easy, fast, and efficient.
Materials and Methods
Growth Conditions
Seeds of Arabidopsis thaliana Landsberg erecta were germinated in soil (Sunshine Mix #1 from McCalif, CA) and grown under continuous fluorescent illumination (80 E m-2s-1) at 23C. Seedlings were irrigated with mineral nutrients (Peters 20-20-20 from Grace-Sierra Horticultural Products Company, Milpitas, CA).
Grafting
Grafting was carried out on plants that had just bolted, approximately 4 weeks after germination. The plants were approximately 10 cm in height. To graft, the primary inflorescence stem of a rootstock was severed horizontally 2-3 cm from the top using a razor blade. The rootstock was inserted approximately 0.5 cm into a capillary replacement tube. The tube was 1 mm in internal diameter and 1 cm in length. The top 0.5 cm of the tube was immediately filled with distilled water using a syringe needle. The primary inflorescence stem of a scion was cut horizontally 3-4 cm from the top and inserted into the capillary tube so that the two cut ends were firmly appressed. The grafted plant was kept under 100% humidity for three days and the humidity was decreased gradually over a period of two to three days. It was not necessary to remove the capillary tube.
Light Microscopy
Graft junctions were fixed in 2% glutaraldehyde and 1% paraformaldehyde in 0.2 M phosphate buffer, pH 7.2, for several days. Tissues were post-fixed in 1% uranyl acetate for 30 minutes and dehydrated in a graded series (30%, 50%, 70%, 80%, 95%, 100%) of ethanol. The tissues were then infiltrated with L. R. White resin (Polysciences) for 24 hours and the resin was polymerized by incubation at 60C for 24 hours. Longitudinal sections of 2-3 microm were cut with a glass knife and mounted on glass slides. Sections were stained with 0.5% methylene blue and visualized under a Leica DMR Microscope.
Results
The developmental sequence of the formation of a graft union has been described as follows (Hartmann and Kester, 1975): 1) Cells at the cut surface of both the scion and rootstock die creating a necrotic plate; 2) Under the necrotic plate, the cambium of both the scion and rootstock produce parenchymal cells termed callus; 3) Cells in the callus differentiate into a new cambium; 4) new xylem and phloem cells are produced in the new cambium establishing vascular connection between the scion and rootstock. To achieve successful graft union, the following criteria were incorporated: 1) Temperature should be high enough for rapid cell division and growth; 2) High humidity is required to prevent desiccation of the thin-walled, turgid parenchymal cells in the callus; 3) The graft junctions should be isolated from possible infection by pathogens; and 4) Firm support is required to allow proliferation of parenchymal cells in the callus.
Using the method described here, we were able to achieve successful grafting at a frequency of approximately 40%. Out of 63 grafts, 26 were successful. Representative plants of a successful grafting are shown in Figure 1. As shown in the figure, the scion produced axillary shoots and flowers that set amounts of seed comparable to ungrafted plants. The scions of grafts that did not take dehydrated and bleached within a few days after grafting.
To confirm that fusion took place at the vascular strands, longitudinal sections of stems at the graft union were examined by light microscopy as described in Materials and Methods. As shown in Figure 2A, new cell divisions occurred below the necrotic plate (indicated by arrows). Figure 2B shows that some of the new xylem and phloem cells have formed a continuous vascular connection between the scion and the rootstock (indicated by arrows). The factors that contributed to successful grafting in addition to the environmental factors are as follows: 1) The diameter of stems of scion and rootstock should be similar; 2) Younger plants are more amenable to making a graft union. A day after bolting is most suitable; and 3) The support for holding the scion and the rootstock needs to be tight-fitting. Capillary replacement tubes of 50 -100 m in volume and 1 mm in internal diameter were ideal. Because Arabidopsis stems do not vary in width greatly, they are amenable to grafting using inflexible supports of fixed diameter.
Figure 1. Examples of a successful grafting.
 Click to see Fig1A
Click to see Fig1A
A. Both scion and rootstock are wild type A.thaliana Landsberg erecta. Picture was taken nine days after grafting.
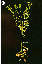 Click to see Fig1B
Click to see Fig1B
B. Wild type A. thaliana Landsberg erecta (scion) was grafted onto line SYR757 (rootstock). Picture was taken three weeks after grafting. Arrows indicate the graft union.
Figure 2. Longitudinal sections of a representative graft union. Both scion and rootstock are wild type.
 Click to see Fig 2A
Click to see Fig 2A
A. Cell divisions initiated below the necrotic plate are indicated by arrows.
 Click to see Fig 2B
Click to see Fig 2B
B. New xylem cells formed in both the scion and rootstock to establish vascular connections are indicated by arrows. Size bars indicate 100 micro meter.
Acknowledgments
We are grateful to Peter Ray for providing literature on grafting, to David Jackson for reading the manuscript, to Ruth Wilson for advice on grafting and to Francis Thomas for advice on sectioning.
Seung Y. Rhee was supported by an NSF Predoctoral Fellowship and by a DOE/NSF/USDA Training Program in Plant Biology. This work was supported in part by DOE grant DE-FG02-94ER20133.
References
Bertaccini, A. and Bellardi, M.G. (1992) A rhabdovirus inducing vein yellowing in croton Plant Pathology 41:79-82
Hartmann, H.T. and Kester, D.E. (1975) Plant Propagation: Principles and Practices, 3rd Ed. Prentice-Hall, Inc. Englewood Cliffs, N.J. pp. 314-427
Janick, J. (1979) Horticultural Science 3rd Ed. W.H. Freeman and Company San Francisco,C.A. pp. 365-371
Jayawickrama, K.J.S., McKeand, S.E., Jett, J.B., and Young, E. (1992) Rootstock and scion effects on carbohydrates and mineral nutrients in loblolly pine. Can. J. Forest Res. 22:1966-1973
Mapelli, S. and Kinet, J.M. (1992) Plant growth regulator and graft control of axillary bud formation and development in the TO-2 mutant tomato. Plant Growth Reg. 11:385-390
Mathews, A., Carroll, B.J., and Gresshoff, P.M. (1992) Studies on the root control of non- nodulation and plant growth of non-nodulating mutants and a supernodulating mutant of soybean. Plant Sci. 83:35-43
Pedalino, M.; Giller, K.E.; and Kipe-Nolt, J. (1992) Genetic and physiological characterization of the non-nodulating mutant of Phaseolus vulgaris L.--NOD125. J. Exp. Bot. 43:843-849
Proebsting, W.M., Hedden, P., Lewis, M.J., Croker, S.J., and Proebsting, L.N. (1992) Gibberellin concentration and transport in genetic lines of pea. Plant Physiol. 100:1354-1360
Tsukaya, H., Naito, S., Redei, G. P., and Komeda, Y. (1993) A new class of mutations in Arabidopsis thaliana, acaulis 1, affecting the development of both inflorescences and leaves. Development 118:751-764.
White, J.W., Montes, C., and Mendoza, L.Y. (1992) Use of grafting to characterize and alleviate hybrid dwarfness in common bean. Euphytica 59:19-25
Zeevaart, J.A.D. (1958) Flower formation as studied by grafting. Mededelingen van de Landbouwhogeschool te Wageningen, Nederland 58:1-88
 Return to Contents Page: Weeds World Vol2(i)
Return to Contents Page: Weeds World Vol2(i)
 Click to see Fig1A
Click to see Fig1A  Return to Contents Page: Weeds World Vol2(i)
Return to Contents Page: Weeds World Vol2(i)